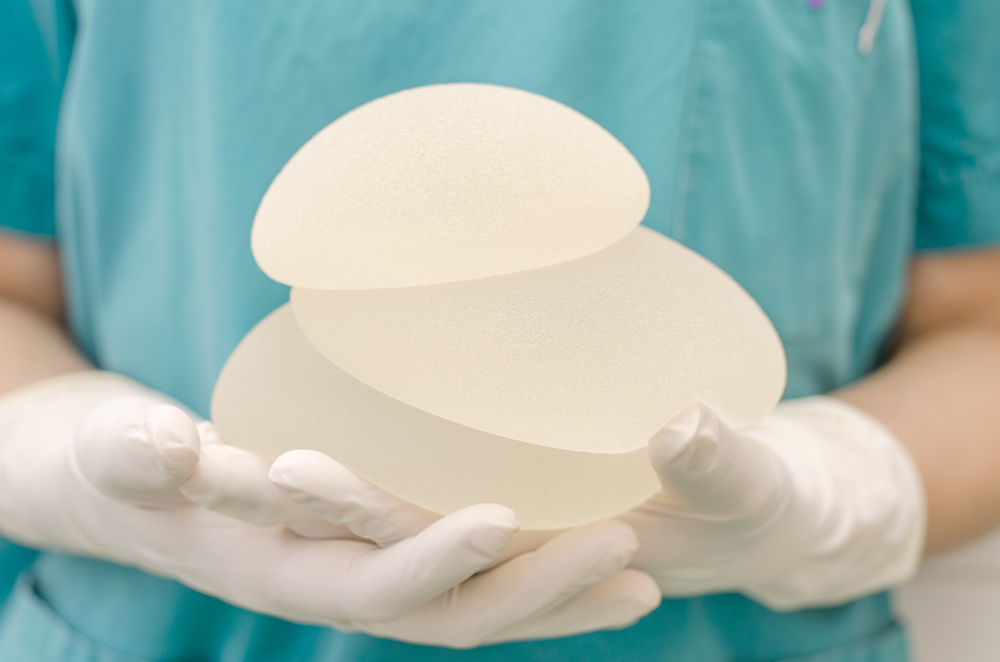
Recently updated global safety information about the connection between textured breast implants and a rare cancer has prompted Allergan to issue a worldwide recall of Biocell textured breast implants and tissue expanders.
In light of this announcement, many women who have undergone breast augmentation may be wondering: Are my breast implants recalled?
Continue reading to learn the specific textured implants that have been recalled and why.
Why Are Certain Breast Implants Being Recalled?
In 2011, the U.S. Food and Drug Administration identified a link between macrotextured breast implants and a rare form of cancer called Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL).
Recently, it has determined that there’s evidence that specific manufacturers’ breast implant products have directly caused significant harm to patients.
Accordingly, they have requested that manufacturers recall these implants.
Are My Breast Implants Being Recalled?
If you have textured breast implants, you may be wondering: Are my breast implants being recalled?
The specific textured breast implants that have been recalled include:
- Allergan Biocell textured breast implants and tissue expanders
- Natrelle Saline-Filled breast implants
- Natrelle Silicone-Filled breast implants
- Natrelle Inspira Silicone-Filled breast implants
- Natrelle 410 Highly Cohesive Anatomically Shaped Silicone-Filled breast implants
The recalled tissue expanders include the Natrelle 133 Plus Tissue Expander and Natrelle 133 Tissue Expander with Suture Tabs.
What is Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL)?
Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) is a rare form of cancer that is linked to textured breast implants.
According to the FDA, there have been 573 cases worldwide and 33 reported deaths.
Symptoms of BIA-ALCL include swelling and pain around the implant, which may occur years after breast augmentation.
Should I Have My Textured Breast Implants Removed?
Currently, the FDA doesn’t recommend implant removal in women without known BIA-ALCL symptoms.
However, it is important to be aware of BIA-ALCL symptoms and seek medical attention from a skilled and board-certified plastic surgeon should you notice any alarming changes.
Learn More About Implant Removal and Replacement
If you would like additional information about the implant recall, please call our office today to schedule a consultation with board-certified plastic surgeon Dr. Navin Singh.
Also, ask about discounted implant removal and replacement.